ni ground state electron configuration|how to write electron configuration : Manila The total number of electrons in nickelis twenty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in nickel in specific rules in different orbits and orbitals is called the electron . Tingnan ang higit pa Welcome to the official website of Tagbilaran City Schools Division, Tagbilaran City, Bohol. This serves as our online portal where you get to know more about us, the things we do and our humble performance. . OO-OSDS-2023-04 GUIDELINES ON THE USAGE OF REGIONAL AND DIVISION OFFICES DEPED PICK-UP VEHICLES. Dec 4, .
ni ground state electron configuration,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of nickel is 1s2 2s2 2p6 3s2 3p6 3d8 4s2. In the nickel ground-state electron configuration, the eight electrons of the 3d orbital are located in the dxy, dyz, dzx, dx2-y2, . Tingnan ang higit paThe total number of electrons in nickelis twenty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in nickel in specific rules in different orbits and orbitals is called the electron . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pahow to write electron configurationScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa
The electron configuration of nickel shows that the last shell of nickel has two electrons and the d-orbital has a total of eight electrons. Therefore, the valence electrons . Tingnan ang higit pani ground state electron configuration how to write electron configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the . Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the . To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of electrons for the Ni atom.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 3p . Here [Ne] refers to the core electrons which are the same as for the element neon (Ne), the last noble gas before phosphorus in the periodic table. The valence electrons Write the expanded and shortened ground state electron configuration for nickel (Ni). Answer. Expanded: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8. Noble Gas: [Ar] 4s .
Nickel is in the 4th energy level, d block, 7th column, this means that the electron configuration will end 3d8 with the d orbital being one level lower than the .

Explanation: Atomic Number of Nickel (Ni) = 28. Electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8. or [Ar], 3d8, 4s2. where [Ar] is Argon with atomic . Mar 23, 2023 Derive the predicted ground-state electron configurations of atoms; Identify and explain exceptions to predicted electron configurations for atoms and ions; Relate electron . Write the ground state electron configuration for Ni 2 +. Answer. Neutral Ni: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8. Ni 2 +: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 or [Ar] 3d 8. Exercise \(\PageIndex{4}\) Write the ground state electron configuration for Rh 3 +. Answer. Neutral Rh: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 7. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of . Ni electronic configuration: [Ar], 3d8, 4s2 Atomic Number of Nickel (Ni) = 28 Electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [Ar], 3d8, 4s2 where [Ar] is Argon with atomic number 18. Chemistry . . What is the ground state electron configuration of the element germanium?
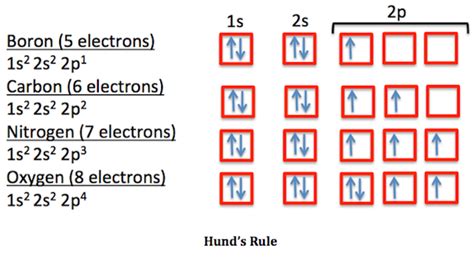
ni ground state electron configuration This means to say that electron pairs only form after all orbital levels have been filled by unpaired electrons. For example, the ground state electron configuration of nitrogen (1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p 2 p orbital.
Nickel at Ground State: Ni: 8 d-electrons = [Ar] 4s 2 3d 8. Nickel with an Oxidation State of +2: Ni 2 +: [Ar] 4s 0 3d 8. Or simply Ni 2 +: [Ar] 3d 8. In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest energy level of n = 4 in this case. So in the ground state the electrons being lost should be the 3d electrons. However the 4s electrons are further from the nucleus so losing the the 2 4s electrons leaves only the third shell electrons making the atoms more stable than losing the 3d electrons. So the somewhat stable electron configuration of Ni^+2 is 1s^2 2s^2 2p^6 .Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy.In each .

Nickel at Ground State: Ni: 8 d-electrons = [Ar] 4s 2 3d 8. Nickel with an Oxidation State of +2: Ni 2 +: [Ar] 4s 0 3d 8. Or simply Ni 2 +: [Ar] 3d 8. In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest energy level of n = 4 in this case.
ni ground state electron configuration|how to write electron configuration
PH0 · ni is the symbol for what element
PH1 · how to write electron configuration
PH2 · full electron configuration of nickel
PH3 · electron configuration worksheet
PH4 · electron configuration ground vs excited
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · current price of nickel per pound
PH8 · Iba pa